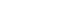Wellness products versus medical devices
Stephen Law
Engineering Software Regulations & Standards Wearable Technology medical devicesToronto-based CardioComm highlights importance of collaboration within emerging sector
EP&T Magazine interviewed Etienne Grima, CEO of Toronto-based CardioComm Solutions Inc., the first company to bring a consumer electrocardiogram (ECG) product to market. In business for 16 years, the firm is described as a software engineering company that specializes in providing ECG management solutions to the healthcare profession around the globe (22-countries). The following is a condensed version of that conversation. (*WATCH VIDEO HERE)
Q. There has been a lot of confusion within the consumer marketplace on the difference between a ‘Wellness Product’ versus a ‘Medical Device’. Can you differentiate?
A. Today there have been many consumer electronic products brought into the marketplace that are designed to be used to assist us in monitoring our health. Usually, these products can be divided into two categories – wellness products and medical device products.
A wellness product is something that doesn’t have to follow or comply to regulations that are applied against devices and tools used to diagnose or interpret the wellness or yourself for effecting treatment or therapy for a condition you may or may not have. For example, I f you were to go to a hospital and have an x-ray, that is done in a hospital setting and the x-ray machine is a medical device that is ‘cleared’ and has function in a certain way that the results are standardized and comparable over time and across different people.
A wellness product is designed to track the user over time. It doesn’t use a standard comparison, that is a medical grade standard. It is giving the user a reader (I.E. number of steps taken). The device provides the user with information related to their activity. Users can choose how to utilize their assessment – whether or not they need to change something in their life. If you are right or wrong, the assessment doesn’t affect the health of the user.
There is an absence of regulatory standardization. There is an absence of imposing standards for measures, because it is a wellness product. Misinterpretation of this device will not endanger the user’s life. We continue to see wellness products make claims that they are going to blood pressure, oxygen level, body temperature, heart rate variability.
We believe that it is important, if you are going to be using devices to monitor your health – (not your wellness) it is only to be done using a medical device.
Q. Describe the role CardioComm plays as a medical OEM?
A. A characteristic of the products that CardioComm brings to market is that they are medically credentialed. We have a pedigree of having multiple country clearances and compliance under ISO Standards. The factories that make our products also have to be under ISO Standards and meet FDA Standards. Our testing of every device, firmware upgrades, changes in any of the electrical components have to follow those regulatory requirements. CardioComm must also follow these requirements, as we supply hospitals direct and we have applied the same standards to the consumer product. That is a distinction that other companies may not be able to provide, because it is expensive and it’s not easy to do. We produce a consumer medical product to the same standards that we produce a hospital-based device, should provide some comfort to the users – whether it’s a consumer or healthcare professional.
From an engineering perspective, wearable medical devices or wellness products have three important components – the hardware, which most people focus on. Such as, does the hardware look nice, does it feel nice – is it expensive?
Then there is a communication protocol associated with it, which involves Bluetooth enabled devices. There needs to be a way of referencing what has been monitored against you and against certain standards. As a medical device, the physician can read the ECG that has been recorded in an environment that is regulated and make a ‘call’ or interpretation or risk assessment to determine what is happening to the medical device wearer.
If that system is not ISO certified (at least) or cleared by Health Canada or the FDA, there can be no certainty that what is being viewed is accurate. The testing has to qualify inputs into the hardware as they are transferred via whatever protocol to the accepting software. That has to be checked against standards. When that information is moved from the user’s environment to an interpreting environment – the integrity of the data as recorded at the point of contact with the user is identical.
This why wellness products are not medical products – because they don’t have to do that.
Q. How important is networking amongst other tech firms within the Medical sector?
A. We are a Canadian company that started in the mid-1990s, and our technology is unique, as there are not a lot of companies in the world that could develop ECG software solutions. Certainly not on a platform like ours – that is device agnostic. Being device agnostic means we depend on other companies to enable our customers to use solutions to monitor their patients. We need hardware partners. We have excellent possibilities to work with Canadian-based hardware manufacturers. A lot of organizations depend on funding to succeed. It’s easier to find funding through American-based organizations. Therefore the thought is you have to be a US-based start-up (or Company) to secure that funding. It’s not necessarily true. It may be easier, but not necessarily true.
A second component to developing new technology is rather than looking for funding to do something from scratch – consider reaching out into your segment community to look for partners, especially if they are in the same geographical area as you. Because you likely to have the same issues as importation clearances, US currency values, etc.
Q. Where do you see wearable tech industry merging with medical OEMs?
A. In the wellness or health medical market, wearable technologies are about monitoring our body.
There are three important components of a medical product, which includes a sensor (hardware), plus receiving software to manage those signals, and then you need a qualified environment in which to measure and assess the meaning of that. Whether the designer chooses to do wearable, tattoo-able, swallow-able, those three elements must be adhered to.
Wearable technologies represent an advancement of the manufacturing process and it is becoming a huge market because it is convenient. If designers can create a wearable consumer product with medical credibility, you will find the advancement of these technologies will move ahead faster because they will be used by doctors under prescription. They will also be used by hospitals.
The technology is exciting, as everyone wants to build a better, cheaper, sexier, more functional device.
Q. Describe the importance that medical devices play.
A. Medical devices are very important in hospital discharges, monitoring, as well as primary and secondary prevention.
Most importantly, these devices have the ability to save lives, increase quality of life and it will save money. The challenge is, largely we the consumers will spend the money on primary prevention. The healthcare system can do it, but they are focused on secondary prevention (illness emergency). Ultimately, it falls on the consumer to take the precautions. This is another reason why access to wellness products is an important ‘first step’, but it is not the best solution. Access to medical devices is really what we need. They are not that different from wellness products. The exception is, medical products are better controlled.
Q. What advice would you give to emerging designers in the medical sphere?
A. One of the challenges is you must have an idea that addresses a need – not something ‘cool’. It is important to get validation from people in the medical market –look for experience and history. Take your idea and bounce it off the right people. Make sure it is an idea that you still believe in, because as an entrepreneur, you must believe in your product. If you don’t, you can’t convince others.
Before proceeding with plans on the new device, you will have to consider the costs to build it, the product’s ease of use, which will ultimately use the product, and what kind of training do they need to do so. After looking at all of the factors, you may be required to consider a partnership. Product developers need to have an idea of what it will look like to get through the progression of your idea – progression into a business, and the progression from a business into an industry.
The best way to protect your idea is often to collaborate. This way you avoid having two like products segmenting the market. When two developers combine their efforts, they gain each other prospective customer base. Instead of having two separate entities struggle and compete, it’s better to build together than build individually. Otherwise, you get two competing products, which mean doubling the marketing costs, legal fees, production costs, and the regulatory reviews. Ultimately, you can get to market faster – together.
Rather than always competing, see if collaboration might work. I believe Canada is a great place to collaborate within, despite the fact that this country is not as big or as overly marketed to as in the USA or Europe. Canada has all the skills required to succeed.