Thornhill Medical Research operates under a unique light
By Stephen Law, Editor EP&T
Electronics Medical designs designs electronics Electronics medical medical Ventilators VentilatorsElectronic design firm led by medical experts takes the road less traveled

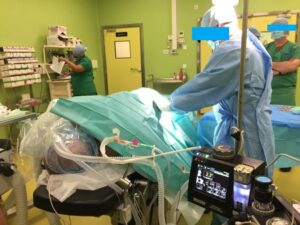
MOVES SLC is an integrated Intensive Care Unit (ICU) that combines and o2 concentrator, o2 conserving ventilator, suction and complete vital signs monitor.
When it comes to designing new electronic devices, Dr. Joe Fisher seems to have come up with the ideal formula – let the end-users create the products. In this particular case, Fisher, an MD with experience in emergency and critical care medicine plus anesthesiology, painstakingly assembled a diverse team of researchers, innovators, scientists, engineers, product specialists and entrepreneurs to create Thornhill Medical Research Inc.
Spun off from the University Health Network in Toronto in 2003, Thornhill Medical has gone on to develop innovative medical technology that is changing the global face of emergency medicine for first responders and the military. In a relatively short time, the company was able to create medical care devices not previously thought feasible, or even possible.
Road less traveled
“If you keep following the same road, you will always arrive at the same destination,” mused Fisher. “So if the company had stuck to high-powered, well known, smart, high achieving scientists, they would have gone down the road well-travelled and ended up making the same products everyone else makes.”
One aspect of product creation that certainly sets Thornhill apart from most other design houses is that the firm only takes on challenges that represent new products—not another version of an existing product—just totally new conceptual entities. Each of its major products: the life support system, MOVES SLC, digital anesthetic vaporizer MADM, treatment of CO poisoning ClearMate, and the blood gas controller RespirAct, are the only ones of their kind.

Dr. Joe Fisher is founder and CEO of Toronto-based Thornhill Medical Research Inc.
Fisher points to another key factor in the mix – he was not a high achieving, classically trained PhD scientist – rather a total scientific outsider.
“But, if I was willing to pay for the ‘research’ myself…well, I could do what the hell I pleased. Indeed, the (medical) journals didn’t know I was a nobody, so I was able to get the work peer reviewed and published,” he adds.
First designs
Fisher was up against it when approached by the military and emergency first responders seeking a device that could deliver fine control of blood gases and a related capability of highly efficient means of delivering inhaled gases. Both requests led to the development of the treatment of carbon monoxide poisoning—an emergency medicine device.
“The high efficiency of oxygen delivery aroused the interest of the USMC (United States Marine Corps.) that wanted to reduce the use of oxygen tanks in the field. At the time they needed these tanks to provide sufficient flow of oxygen to satisfy the requirements of a patient who was breathing hard,” Fisher says.

MADM is a portable inline direct injection gas anesthesia vaporizer, capable of delivering isoflurane (ISO) and sevoflurane (SEVO) gas anesthesia safely and accurately.
Thornhill delivered on a proof of concept which boosted oxygen levels without tanks. Then came what Fisher refers to as ‘feature creep’. Marines asked if we could put physiologic monitors such as ECG, blood pressure, and gas sensors, on our device; provide a monitor to display the values; add a ventilator, that could be run from the monitor; and add suction capability.
“Once we could do that, they asked if we could develop a small anesthetic delivery system suitable for the field. Each one of which, the nay sayers pointed out, was totally out of our expertise, off the focus of the company, overwhelmingly difficult, time consuming and expensive to do. No way we should do it,” Fisher chortled. “But, to all of which we answered, ‘yes, of course.’ And, hence we had a company.”
The team
On his journey, Fisher has moved in lockstep with a medical brain trust comprised of Drs. Ludwik Fedorko, Steve Iscoe, Jim Duffin and a group of founding engineers that managed to commercialize many of their ideas and research. The cluster of P.Eng types is comprised of a diverse ecosystem that includes: Kevin and Brian Kowalchuck, electrical engineers; Kevin Ramkalewan, electrical engineering student; Drew Miller, electrical and software engineer; Cliff Ansel, engineering science; Veso Tijanic and Eduardo Massionis, mechanical engineers; George Volgyesi, biomed engineer.
Conceptualizing
Fisher explains that the actual work of the company is conducted in a series of leaps of faith: into a new product, into new science, and new engineering, with the result not known until all the components are stretched and molded this way and that in an attempt to get to a practical working product.
“Having made the damn thing, the next challenge is to get buy in – convincing people that the new device works better than what they are using and worth the trouble of changing,” says Fisher. “Fortunately, for our first set of products it has been inevitably true that it is better than their current practice. But, we find great reluctance in people to nevertheless abandon their practiced approach.”
MADM portable anesthesia vaporizer uses any source of ventilation in both controlled and challenging environments over a broad operating temperature range.
Collaboration
Collaboration through the design process is essential, according to Fisher, as the design team is multidisciplinary and require high level of coordination. He adds that collaboration between different engineering disciplines is the challenging process that requires the team members to step outside their traditional engineering area in order to bridge the gap between the disciplines.
Even more challenging is the collaboration between the Medical Science and Physiology team and Engineering Teams. The physiological parameters need to be understood and transferred to the engineering terms. The physiology systems need to be modeled using electromechanical principles and later designed and build in the physical device. This process requires the engineers with the medical science background and ability to constantly learn and develop.
The next level of collaboration is achieved through the project management where the activities are synchronized between the groups and individual contributors.
Home made

Used by multiple international armed forces for casulty care during transport, MOVES SLC is an integrated Intensive Care Unit (ICU).
When it comes to actually creating these medical devices, Thornhill develops and designs its products at the Toronto office. Initially, the firm operated as a design and development company. It soon developed its own internal resources to primarily handle the new product development. To be effective, Fisher realized that the company needed to invest in prototyping techniques. Thus, the firm purchased two Fused Deposition Modeling (FDM) machines 14-years-ago when the technology was still new. This, combined with the prototyping shop that had two CNC machines and other prototyping equipment, helped Thornhill effectively produce prototypes and accelerate design process.
“In parallel we built the prototyping team that was the core force for the future development of our manufacturing team,” Fisher says. “We looked at contract manufacturing options, but realized that due to the product specifics – we were better off developing our manufacturing capabilities.”
Fisher says his team works closely with its component suppliers and performs final assembly and end-testing in house at the Toronto facility.
“Our manufacturing capabilities will strengthen with our recent move to the new (Markham ON) office, which is better suited for manufacturing and material flow.”
Making medical
The complexities of creating a certified medical device, versus that of creating a simple wearable consumer device are amplified by the human interface and the life dependence to the medical device, according to Fisher.
“The margin of error and risk of failure make the medical device highly challenging,” says Fisher. “Making the Life Support System creates a level of responsibility that is not necessarily present with some other devices.”

ClearMate – a portable and patented device that lets doctors treat carbon monoxide (CO) victims as effectively as hyperbaric oxygen.
Fisher points out that the medical device environment is highly regulated. The facilities and processes are held by the higher standards and constantly evaluated. The regulations are constantly evolving – ensuring that the products keep up to date with the new standards.
The product risk evaluation is very rigorous and present at any step of the product life.
“That is what makes it unique in comparison to the other products,” he says.
Fisher lists off some of the biggest hurdles or challenges in having a medical device certified.
- Strict and rigid regulations
- High cost of the certification process
- Constantly changing requirements
- Bureaucratic approach
Working on multiple complex medical devices simultaneously turns out to be the biggest challenge for a relatively small company like Thornhill Medical.
“It is our biggest operational challenge,” he says. “Since we cannot afford to have dedicated team for a single product, we need to have people that are ‘light on their feet’ – able to quickly move to the new challenge. We are working on the long-term scheduling, prioritizing and we communicate the expectations in order to effectively manage multiple products.”
Acute designations
Bursting with pride over the work they have achieved thus far, Fisher proudly states that Thornhill Medical will become the world standard for life support systems, in addition to becoming the source of the world-wide accessible treatment for carbon monoxide poisoning. Fisher asserts his group will also be a key component to the gold standard for the use of MRI for the prediction of stroke and heart attacks, and tumor virulence.
Fisher shares what he feels are three of his firm’s biggest accomplishments over the years. Without any doubt, first on his list was Thornhills Medical’s capability of taking three disruptive products from whiteboard drawings, through prototypes, products, regulatory approval, manufacturing and distribution to service in the field of the world’s most advanced and discriminating customers.
“Second, has been the development of scientific and engineering breakthroughs that will spawn and support whole new fields of scientific discovery and technological wizardry,” Fisher says. “Third, is survival and accomplishments for 15 years at our own devices—bootstrapped with ‘friends-and-family’ support only.”
Moving Forward
Fisher stressed the importance of Thornhill’s engineering team to continue inspiring innovation – in whether it is developing a new device or redeveloping or re-designing an existing product.
“Our engineers are not only the actors on the stage, but they have been here long enough to have conceived of the play, written the dialog and music and helped stage the production,” Fisher says. “This gives them, and the rest of the company, great depth in continuing to innovate and improve our unique products. The older, grayer the head, the more we cherish it.”