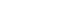EU SCIP database – Reporting obligations for electronics
By Walter Jager, principal consultant, ECD Compliance, Ottawa
Electronics Regulations & Standards Engineering REACH REACH SCIP database SCIP databaseTo come into effect for manufacturers, importers and distributors (duty holders)
On January 5, 2021 — in less than a year — the reporting obligations for the European Substances of Concern in Products (SCIP) database comes into effect for manufacturers, importers and distributors (duty holders). The reporting applies to products placed on the EU market that contain a REACH Candidate List SVHC above 0.1% w/w in any article in the product.
There are still several common EEE applications where SVHCs are needed and cannot be easily substituted, especially given that lead (Pb) is now on the REACH Candidate List. This means that many complex EEE products will need to be submitted into the SCIP database. If they aren’t in SCIP, they’re easy enforcement targets for customs and surveillance authorities.
The database poses difficult challenges for manufacturers and suppliers given complex supply chains, data requirements that go beyond what most manufacturers have collected from their suppliers and no volume thresholds for small and medium sized manufacturers.
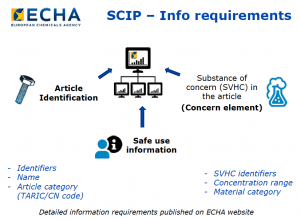
FIG 1: SCIP Information Requirements (Source: ECHA, 2019).
The mandate for SCIP comes from Directive (EU) 2018/851 revising the EU Waste Framework Directive (WFD). It requires ECHA to develop and operate the database and directs EU Members States to require the duty holders to submit information about their products into the database. The EU is imposing this new obligation in part due to what it sees as poor REACH Article 33 compliance.
Information Requirements
The SCIP data requirements include article identification, safe use information, and information about the substance of concern (SVHC) (see Figure 1). This includes identifying the component articles that contain SVHCs. Several industry associations and standards development groups have been working with ECHA to help them understand the complexities of global supply chains, multi-sourced BOMS, and concerns about confidential information. Some of the efforts have been successful with ECHA changing some data fields to optional; but there is still information that may not be readily available.
Mandatory information about articles includes article name, primary article identifier, article category (CN tariff code), safe use instructions, number of units, and whether or not the article was produced in the EU. Information about the substance includes the version of the Candidate List used in the assessment, the Candidate List entry, concentration range and the material or mixture category. Optional information such as other names, identifiers, size, weight, and colour characteristics, pictures, disassembly instructions may be provided.
In an attempt to reduce concerns about confidential information (CBI), ECHA has taken steps to hide certain information from public access, especially information that could be used to identify suppliers.
SCIP data format and its importance to manufacturers
Figure 2: SCIP Information Requirements and Format.
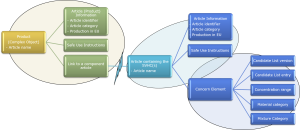
The SCIP data format is implemented as a series of XML schemas and picklists (drop down lists) in the ECHA IUCLID 6.4 software. ECHA is also providing an online web interface into SCIP to simplify the process for manufacturers with only a few submissions and a system-to-system portal for bulk uploads.
The “Link to a component article” (Figure 2) allows a complex object to link to a supplier’s part (if already in the SCIP database) or otherwise a component article submitted by the manufacturer.
Awareness of the SCIP data format is important to manufacturers because it provides insight into the information needed from their supply base. For example, a specific data element may need to include a data type, use a specific format or correspond to an entry in a picklist. A good understanding of what data should be requested from suppliers is crucial for data quality, reducing effort and avoiding multiple requests to suppliers.
ECHA is currently trialling a prototype SCIP database with the SCIP IT User Group (ECD Compliance is a member and is participating in the trial). They expect to have the production database deployed in October 2020, allowing duty holders to make their submissions before the January 5, 2021 deadline.
Moving forward – Collecting supply chain data
Several industry associations continue to oppose the broad content requirements for SCIP and court challenges are being considered. But this isn’t likely to affect legal obligations in the short term; in the meantime, manufacturers with products that potentially contain REACH Candidate List substances should be preparing their supply base. The time that remains before manufacturer obligations take effect is minimal given the task. Eliminating SVHCs from products is ideal, but there are products and applications for which this is not yet technically feasible.
In addition
Additional information on the SCIP database and reporting obligations may be found on the ECD compliance new blog (https://rohs.ca/news/?s=SCIP ) and guidance on using the IEC 62474 material declaration standard for SCIP data collection may be found on the IEC62474 blog (https://rohs.ca/iec62474/ ).