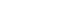Vena Medical marks first procedures with medtech device
EP&T Magazine
Electronics Embedded Systems Medical medicalTechnology developed to remove clots in brains of stroke victims
Vena Medical, a Kitchener ON-based developer of cutting-edge neurovascular devices, has successfully treated its first five patients in the world using the firm’s medical tech device – the Vena BDAC – at London Health Sciences Centre (LHSC) University Hospital and The Ottawa Hospital (TOH).
Launched by two Waterloo Engineering graduates, Vena Medical grew out of a Capstone Design project by Michael Phillips and Phillip Cooper when they were classmates in the mechanical engineering program, earned Health Canada approval earlier this year for hardware it calls the Vena Balloon Distal Access Catheter (BDAC).
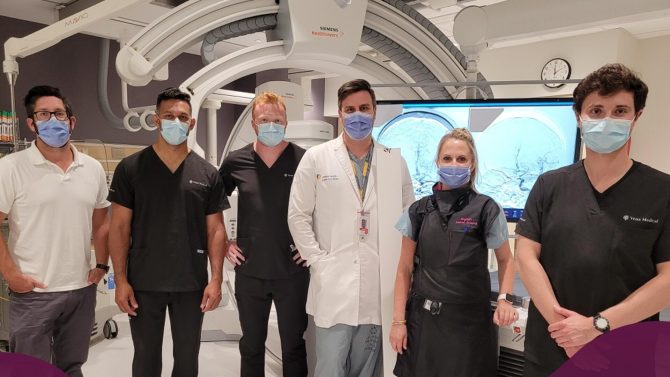
From left, Jon Collier, Adam Karamath, Michael Phillips, Dr. Michael Mayich, Elizabeth Cesarin, and Phillip Cooper following the First-In-Human use of the Vena Balloon Distal Access Catheter at LHSC.
The Vena BDAC combines the balloon guide catheters and distal access catheters that are currently used in thrombectomy to remove clots from the brains of stroke victims. Combining these two devices allows the clinician to get the balloon much closer to the clot, which is shown to improve key metrics like First Pass Success Rate, allowing removal of the clot on the first try and leading to significantly better patient outcomes. This also reduces the number of devices to treat each patient and therefore the cost of the procedure.
“The BDAC isn’t even comparable to the competitors,” describes Dr. Sachin Pandey, who completed the procedure alongside Dr. Michael Mayich in London, ON at the London Health Sciences Centre-University Hospital. “I easily pulled back the Stentriever and clot all the way through the lumen of the BDAC with zero resistance. You can’t do this with the current devices on the market.”
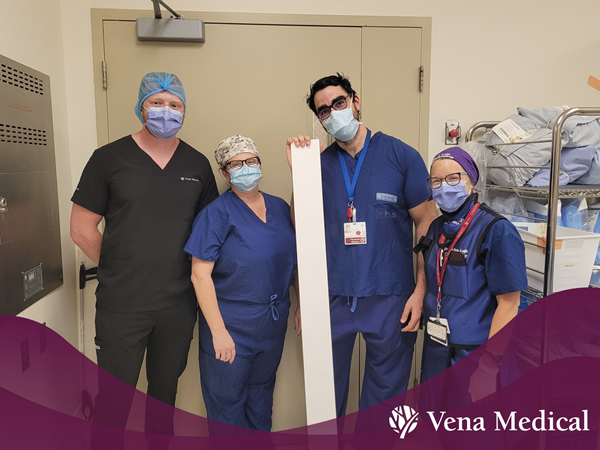
From left, Michael Phillips, Lisa Weatherill, Dr. Robert Fahed, and Dr. Jelka Lujic following the First-In-Human use of the Vena Balloon Distal Access Catheter at TOH.
“We’re proud to have the first patients to benefit from our Canadian developed technology within driving distance of our office, in the hands of world-class physicians. This is cutting-edge technology that would normally need to be launched somewhere far from here, but we’re excited to make an impact locally before we make an impact globally” says Michael Phillips, the CEO of Vena Medical.
“The Vena BDAC is a category-defining device and we’re excited to be the first in the world to evaluate its performance. Each case has been successful so far with the BDAC getting the clot out on the first try, every time” said Dr. Robert Fahed of The Ottawa Hospital.
The Vena BDAC was developed out of the company’s flagship product, a tiny camera – billed as the world’s smallest – to allow doctors to see inside veins and arteries during stroke surgery. The company is now based at the Medical Innovation Xchange in Kitchener and has eight employees.
For more information on Vena Medical visit www.venamed.ca.