Considering the differences between medical and consumer wearables
By Travis Stevens, PEng., MSc., vice-president engineering, Orpyx Medical Technologies Inc.
Medical medical electronics medical electronicsCalgary-based Orpyx Technologies Inc. explores the differences in design, commercialization and potential between medical and consumer wearables
Our earliest ancestors first created wearable technology the instant they covered themselves as a means of protection from their environment. From that humble beginning, wearable technology has continued to evolve, powered by the discovery of new materials, components (both mechanical and electronic) and the creation of new manufacturing techniques.
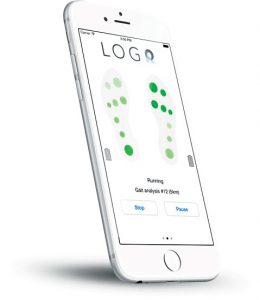
Orpyx LogR – Real-time measurement of plantar pressure for research. No tether, computer, or lab is required.
Even the term wearable has undergone an evolution itself. Over the past decade wearable has become a noun used to describe devices and textiles enhanced with sensors and electronics that measure and interact with their users and their environment. As it currently stands, wearables have almost exclusively been targeted at the consumer market, with the bulk of these devices being classified in the subcategory of fitness and activity trackers. However, there are new and novel medically-focused wearable devices emerging from the shadow of the consumer space. These new medically focused wearables, such as the Orpyx SurroSense Rx, are able to leverage both the awareness and acceptance enjoyed by consumer oriented devices and incorporate the technology and manufacturing used in the consumer products to make highly integrated, special purpose devices.
The following article explores some of the differences in the medical and consumer wearables as it relates to design, commercialization and potential.
Largest difference in commercialization is regulatory requirements
The single largest difference in the commercialization of consumer and a medical wearable is the regulatory requirements that must be met. Every country in the world has its own regulatory framework and approvals process, and though there is some commonality between them, success in any given market requires understanding and conforming to the regulatory framework in place for that country. The largest organizational impact of regulatory compliance will be the need to have a quality management system in place. The industry-accepted quality system for medical devices is ISO 13485, and implementation of a 13485 quality system will affect every part of the business involved in the realization and commercialization of the device.
The ISO 13485 quality system, and for that matter, health regulatory bodies, are very focused on minimizing the risk to patients. The primary mechanisms for risk mitigation are risk assessments. From a design perspective, a risk assessment should be done at the outset of the design process, and continued throughout the product lifecycle to ensure that risks can be mitigated as early, and as cost effectively as possible. Also important to the quality system is the need to show requirements traceability; in other words, can a line be drawn from the creation to realization of a requirement in the product. Requirements traceability is nothing new to product development, however, what is important in the medical wearable context is the need to have evidence that shows the above requirements’ traceability.
Key differentiator is to have evidence that validates any claims made
Another key differentiator between the medical and consumer wearable market is the need to have evidence that validates any claims made as it relates to the device. In the consumer world, there have been a number of companies defending themselves in class action lawsuits because their products did not live up to the claims being made, however, these lawsuits were only initiated once the products had already been on the market and enjoyed widespread adoption. There was no requirement for there to be evidence supporting the product claims prior to the sale of the product.

Identify trends and track patient progress. The SurroSense Rx system is a tool to help manage and track complications.
On the other hand, medical wearables are required to have evidence that supports the medical claims being made by these devices, and the only way to gather this evidence is through trial. The complexity and scope of the clinical trial will be related to the nature of the claims being made, and in the end it will be up to the regulatory body to determine if there is sufficient evidence to validate the claims. The time and effort required to gather this evidence is something that a consumer-oriented company could easily underestimate if it attempts to pivot its technology to a medical application – it should anticipate major burdens in both regulatory approval and go-to-market efforts.
Components & algorithms must have the accuracy required to fulfill claims
Related to the need to provide evidence for the efficacy of the device, is the need to create a device that is accurate. As an example, the accuracy of consumer activity trackers has been studied by a number of different institutions, with the reported accuracy varying depending on the activity being studied. Also, a quick review of the top consumer wearable websites reveals that no concrete claims are made as to the accuracy of the device, and to be fair, no one is being put at risk if an activity tracker under- or over-reports the number of steps that an individual has taken. In contrast, a medical wearable must be designed to be accurate because the consequences of an inaccurate measurement could result in harm to the user. Even though medical wearables are able to leverage many of the same constituent parts as the consumer products, additional care must be taken to ensure the components and algorithms have the accuracy required to fulfill the claimed purpose of the device.
One of the interesting promises of medically-oriented wearables that combine the usability and connectivity of a consumer wearable with the accuracy of a medical device is the ability to continuously monitor patients and gather data that was previously unavailable to healthcare practitioners. However, conducting analysis on health-related data is not without its own set of challenges. First and foremost, privacy is the major concern when it comes to medical data, and many countries have strict laws as it relates to the handling of patient health information. Violation of these laws can result in serious fines, so before any of this information is collected, it is critical to understand the privacy laws of the country in which the devices are used and to address all the technical issues as it relates to the collection, transmission and storage of patient health information.
Advances in wearable tech opens up new applications
The second issue with the data generated by medical wearables is the sheer volume of it. Luckily, tools and techniques such as machine learning and infrastructure such as cloud computing are making it possible to collect and analyze this large volume of data in a way that was never possible before. Collection of this data will improve the quality of care for patients a number of different ways, it will make it possible for healthcare practitioner to monitor patients outside of the hospital, and, if necessary, intervene based on the data being recorded. It will also allow for better insights as it relates to disease progression.
Advances in wearable technology has opened up a number of new applications, including the development of medically-oriented wearable devices. The potential of these medically-oriented devices is significant by combining highly accurate data collection with high connectivity and bidirectional communication. However, bringing these types of devices to market is not without unique challenges. It is essential to comply with the regulatory environment of the market, and to gather sufficient, objective evidence that supports the claims made of the device’s benefits and performance.
Additionally, ensuring patient privacy is critical in the collection, transmission and storage of data. Even given these challenges, the future of medical wearables looks extremely bright and I look forward to continuing to on the leading edge of their development.